Department of Analytical Research and Development,
Merck & Co., Inc., Rahway, NJ, USA
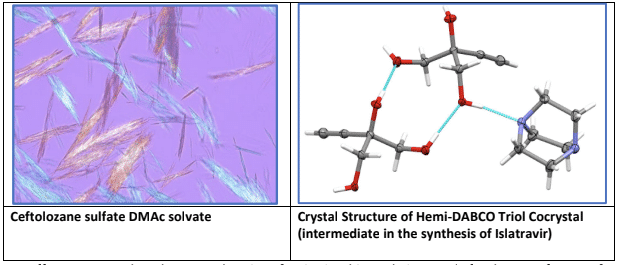
The role of the crystalline solid phase is often an enabling feature in the development of robust, efficient, and scalable pharmaceutical processes. While the idealized synthetic process may be one where raw materials enter and then the finished API emerges with minimal isolations along the way, crystallization remains a fundamental unit operation enabling both purity control and supply chain flexibility. Crystallization, however, also can be an enabling feature of greener processes. Three exemplary instances from Merck’s Process Research & Development teams will be presented. In the commercial synthesis of ceftolozane sulfate — the active ingredient in Zerbaxa, a β-lactam antibiotic — Merck was able to leverage crystallization of a key solvate form of the API to enable realization of myriad chemistry and process development innovations. This breakthrough led to the receipt of the 2019 Green Chemistry Challenge award for greener synthetic pathways. There had been a long-held idea that the purification of β-lactam antibiotics required chromatography. The discovery of a dimethylacetamide solvate of ceftolozane sulfate enabled the process development team to eliminate both chromatography and a nano/diafiltration sequence in the implementation of a single step anion metathesis/crystallization. The unnatural, alkyne-containing nucleoside analog islatravir is synthetically accessed through a three- step biocatalytic cascade starting from the triol, 2-ethynylglycerol, a true innovation of green chemistry.1 Crystal form became a critical feature of the overall process development, at both ends of this cascade. Various crystal forms of the triol starting material were profiled to find a solution that overcame both safety and supply chain liabilities. Two suitable options (a DABCO cocrystal and a crystalline TMS protected version of the triol) were found to enable an efficient transition from traditional chemical synthesis into the cascade. Coming out of the cascade, it became necessary to leverage different crystal forms of the API to both achieve purging of accumulated impurities as well as to deliver API with the target physical properties required to support different clinical formulations. A rapidly emerging pharmaceutical modality, the antibody drug conjugate (ADC) provides unique process development challenges. Many of these therapeutics possess highly flexible synthetically derived linkers that are thermodynamically (or practically) inaccessible as crystalline solids. The Merck team has creatively discovered that the application of crystalline solid supports (such as cellulose or celite) can be readily leveraged in these cases to vastly improve physical properties. This strategy is enabling the team to take advantage of better, more robust and greener chemical transformations thereby and reduce the reliance on costly, inefficient and high-solvent use chromatographic separations.
 1. Huffman, M.; Fryszkowska, A.; et al Design of an in vitro biocatalytic cascade for the manufacture of
islatrvir, Science 2019, 366, 1255-1259.
2. Rummelt, S.; Qi, J. et al. Development of an Efficient route to 2-Ethynylglycerol for the Synthesis of
Islatravir, ChemRxiv 2021.
1. Huffman, M.; Fryszkowska, A.; et al Design of an in vitro biocatalytic cascade for the manufacture of
islatrvir, Science 2019, 366, 1255-1259.
2. Rummelt, S.; Qi, J. et al. Development of an Efficient route to 2-Ethynylglycerol for the Synthesis of
Islatravir, ChemRxiv 2021. 





